Our projects
Interferon is produced by infected cells following the detection of pathogenic viruses and bacteria and is the first line of defence against infection. Interferon induces the expression of several hundred genes, names interferon-stimulated genes (ISGs) both in infected and neighbouring cells. The products of the ISGs, in turn, allow the establishment of a so-called antiviral state, which is able to prevent, or at least limit, viral replication. Most viruses, including influenza A virus, the Human Immunodeficiency Virus type 1 (HIV-1) and SARS-CoV-2, are highly sensitive to this antiviral state and unable to replicate efficiently in cells that have been pre-exposed to IFN.
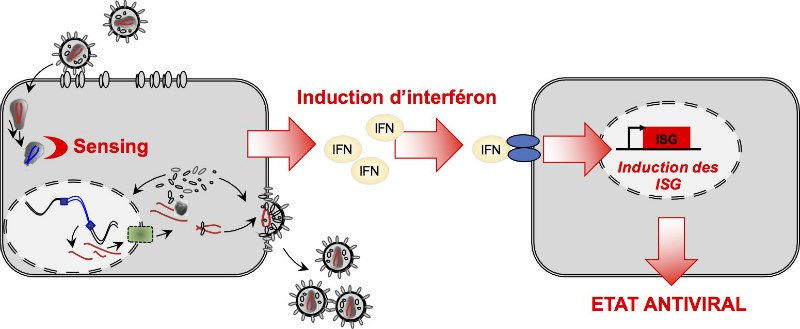
The dynamin-like, high-molecular weight GTPases MX1 and MX2 play a significant role in the interferon-induced inhibition of viral replication. Human MX1 is a restriction factor of broad antiviral activity, able to inhibit a great diversity of RNA and DNA viruses at different stages of their life cycles. The activity of MX2 seems narrower and has so far been restricted to HIV-1 and some primate lentiviruses. MX2 prevents HIV-1 DNA nuclear import and integration. Both MX1 and MX2 seem to recognize and interact with key components of viral nucleoprotein complexes to prevent viral replication, however their detailed mechanisms of action remain to be understood. Other antiviral ISGs inhibiting HIV-1 and influenza A virus have been identified, however, our preliminary data show that additional genes remain to be identified. We have recently identified the short isoform of NCOA7, which is interferon-inducible, as a new antiviral factor preventing endocytosis-meditaed viral entry.
Our lab, named Interferon and antiviral restriction, which has been established in January 2015 at the CNRS institute of research on infection of Montpellier (IRIM, ex-CPBS), aims at identifying new cellular effectors of the antiviral state (using, among other approaches, whole-genome scale CRISPR/Cas9 screens) and at characterizing the molecular mechanisms involved in their antiviral activity.
Our main projects are the following:
- Understanding the antiviral activity of MX, NCOA7 and DDX42 proteins
- Physiological role and potential involvement in innate immune signalling of MX proteins
- Identification and characterization of new interferon-induced, antiviral effectors against HIV-1 and influenza A virus and other human pathogenic viruses, including SARS-CoV-2
- Identification of new genes regulating influenza virus and SARS-CoV-2 replication and search for new antiviral compounds
Since March 2020, we’ve also been studying SARS-CoV-2: we’re chacterizing the inhibitory effect of interferons, identifying cellular regulators of replication through whole-genome CRISPR screens and screening molecules to help identify new therapeutical avenues.
Key words
Interferon, antiviral restriction, HIV-1, influenza A virus, SARS-CoV-2, innate immunity, signalling, genetic screens, CRISPR/Cas9
Team members
- Caroline Goujon – Team leader, INSERM tenure researcher (DR2)

Caroline studied at at the Ecole Normale Supérieure (ENS) of Lyon. She did her Master’s and PhD in Dr Jean-Luc Darlix’s laboratory (Inserm U412/U758; Sept. 2002-Jan. 2008), under the supervision of Dr Andrea Cimarelli. She studied the restriction of HIV-1 infection in human dendritic cells and discovered for the first time that HIV-2/SIV Vpx was able to relieve this block. She then did a long postdoc in Prof. Michael Malim’s laboratory at King’s College London (Feb. 2008-Dec. 2014), where she was awarded a Marie Curie Intra-European Fellowship to study the host cell responses to HIV-1 infection. She became interested in the relationships between HIV-1 and the interferon system and characterized in depth the interferon-induced restriction of HIV-1 infection. She notably identified human MX2 as one of the major interferon-stimulated genes preventing HIV-1 infection. In 2014, she was awarded the Andy Kaplan prize and obtained both an INSERM researcher permanent position and an ATIP-Avenir grant to move back to France and start her own group. She has been working at the CNRS IRIM institute (which was formerly called CPBS) in Montpellier since January 2015. She has obtained in 2016 grants from Sidaction and the ANRS. She has been awarded an ERC Starting Grant in 2017 to study the mechanisms of interferon-induced antiviral restriction and mechanisms (ANTIViR project). In 2020, the lab obtained several fundings to work on SARS-CoV-2, MUSE (Montpellier university), from INSB, La fondation CNRS, the ANR-RACOVID, in order to identify and characterize anti-SARS-CoV-2 molecules, characterize the host cell responses to infection and identify the cellular regulators of viral replication through CRISPR screen. In 2020, C. Goujon has also been awarded a PRC ANR grant to study NCOA7’s mechanism of action and role in vivo, in collaboration with M. Blaise (IRIM) and M. Wencker (CIRI). In 2023, C. Goujon has been awarded an ERC CoG for the 2022 call (project InVIRium), to study the interactions between respiratory viruses and primary airway epithelia. In 2023, Caroline was also awarded Horizon Europe collaborative funding as part of the APPEAL project (2024-2028), which aims to develop new antivirals, and was promoted to DR2 by Inserm.
- Olivier Moncorgé – Permanent Scientist CNRS (IR, from Dec. 2021) (former postdoc ATIP – ANRS – ERC Feb. 2015-Dec. 2020)
After working as the molecular biology group leader in a French biotech company (Aptanomics SA, Lyon) and as an engineer (IE) on HIV-1 translation at the Ecole Normale Supérieure of Lyon, Olivier did his PhD and a 2-year postdoc in Prof. Wendy Barclay’s laboratory, at Imperial College London. Olivier worked on the molecular basis of host range restriction of avian influenza polymerases in mammalian hosts. During his first post-doc, Olivier was part of the European consortium ANTIFLU, aiming at identifying new inhibitors of influenza virus replication. Olivier also studied MX1 protein activity against influenza A virus. Olivier has joined the lab in Feb. 2015, thanks to the support of the ANRS (2-year fellowship obtained by C. Goujon in connection with the ATIP-Avenir grant, and 1-year fellowship renewal obtained by O. Moncorgé). Olivier has been working on the ERC ANTIViR and FluAttack projects since 2018 and 2019, respectively, and led all the influenza 1 virus-related topics of the lab. Thanks to his expertise on respiratory viruses, the team was able to rapidly start working on SARS-CoV-2 back in March 2020. Olivier notably led all the screening projects on SARS-CoV-2. His contract in the lab ended in Dec. 2020 and this year, Olivier was self-employed and a consultant on respiratory viruses. In 2021, he has obtained a permanent scientist position (IR) at the CNRS as a respiratory virus expert. Within IRIM and in parallel to his work in the team, Olivier has set up a technical platform for the study of respiratory viruses (webpage coming!). The purpose of this platform is to share Olivier’s expertise on respiratory viruses and in particular on influenza A and B viruses and on coronaviruses, to train people from the institute to their handling in BSL2 and BSL3 settings, to develop innovative viral reporter systems and to share them with the scientific community in the form of collaborations or services, in particular for antiviral molecule screening.
- Mary Arnould – Lecturer (Montpellier university)

“Normalienne agrégée” (ENS-Cachan), Mary did her PhD thesis Paris XI university in immunology and has been teaching since then at the IUT of Montpellier. Mary has joigned the team in March 2019 and uses her strong expertise in biochemistry to study MX1, NCOA7 and DDX42 antiviral proteins, in close collaboration with M. Blaise (IRIM).
- Antoine Rebendenne – Post-Doc

« Normalien agrégé » in Biochemistry and bioengineering from the Ecole Normale Supérieure Paris-Saclay (ex ENS Cachan), Antoine did his Master 2 in Fundamental Virology (Sorbonne University/Pasteur Institute). After carrying out several internships both in France (in our team during summer 2016) but also in London (Imperial College London, UK) in 2017 and in New York (Rockefeller University, USA) en 2018-2019, Antoine came back to do his master’s thesis in our team. Under the supervision of Caroline, Antoine worked on the characterisation of a new interferon-induced antiviral protein, NCOA7, and on SARS-CoV-2 after the end of the first lockdown (May 2020). Antoine completed his thesis both in the team and in cotutelle with Mélanie Wencker (CIRI, Lyon), with whom he studied the role of NCOA7-AS in vivo. Antoine defended his thesis in November 2023 and returned to the team as a post-doc in February 2024 to lead the ERC InVIRium project.
- Elodie Bishop. PhD student (and former Research assistant CNRS 2021-2022).

Elodie is a MD-PhD student from the ENS of Paris. She has temporarily interrupted her medical studies to focus on research in Virology. She completed Fundamental Virology Master 2 at Sorbonne University and Institut Pasteur in 2021. After obtaining a CDSN, she joined the team as a PhD student in September 2022. She has since been working on the characterisation of a novel inhibitor of the SARS-CoV-2 and HCoV-229E coronaviruses.
- Alice Trausch. CIFRE PhD student.
 Alice is doing her PhD between the team and the Voxcan private company. The goal of her thesis is to develop novel reverse genetic systems for respiratory viruses (Influenza and SARS-CoV-2) under the supervision of Olivier Moncorgé and to set up primary cultures of airway epithelia.
Alice is doing her PhD between the team and the Voxcan private company. The goal of her thesis is to develop novel reverse genetic systems for respiratory viruses (Influenza and SARS-CoV-2) under the supervision of Olivier Moncorgé and to set up primary cultures of airway epithelia.
- Jimmy Cadènes – PhD student

Jimmy graduated from AgroParisTech engineering school, and got his Master 2 in Fundamental Virology at Institut Pasteur/Université Paris-Cité. He completed a 4-month internship at Institut Pasteur in 2022 on the development of reverse genetics and pseudotyping systems for a bat vesiculovirus, and a 6-month internship at ENS Lyon on the impact of herpes simplex virus type 1 on the ribosome interactome. He also completed a 4-month “field virology” internship at the Institut Pasteur du Cambodge, on an avian coronavirus (infectious bronchitis virus). He then joined the team for his Master 2 internship, during which he worked on the mechanism of action of the broad-spectrum antiviral protein MX1 against various human viruses: La Crosse virus (LaCV), vesicular stomatitis virus (VSV) and influenza A virus (IAV). Thanks to a funding from the Fondation pour la Recherche Medicale (French Foundation for Medical Research, FRM) Jimmy joined the team in September 2024 for his PhD thesis on the restriction of influenza A virus by respiratory epithelial cells, with a focus on the MX1 protein.
- Issy Fernanda Hincapie Mena – Study engineer

Fernanda first joined Caroline Goujon’s team in January 2024 as part of her Master’s internship in Biology, specializing in the Microbiology, Immunobiology, and Infectious Diseases (MIDI) program at the Université Grenoble Alpes (UGA). During this internship, she worked with Olivier Moncorgé and Alice Trausch on developing reporter influenza viruses for recently circulating strains using reverse genetics techniques. In September 2024, Fernanda joined the team as a research engineer for the APPEAL project (Antivirus Pandemic Preparedness European Platform). She contributes to optimizing CRISPR-based genome editing techniques to validate genes of interest involved in the replication of major pathogenic viruses. This work aims to better understand virus-host interactions and aligns with the project’s overall strategy of identifying key host factors and developing broad-spectrum antivirals against emerging or re-emerging pathogenic viruses
- Guorong Sun – Post-doc
Guorong completed his PhD at the Institute of Virology, Hannover Medical School, Germany, in the laboratory of Prof. Dr. Abel Viejo-Borbolla, and subsequently did a short postdoc in the same lab. During this period, Guorong’s research primarily focused on the neurotropism mechanisms of herpes simplex virus. Since July 2024, Guorong has joined the Goujon Laboratory as a postdoc to work on the ERC-funded InVIRium project.
- Justine Lagisquet – Post-Doc
Justine performed her PhD thesis within the laboratory of Professor Thomas Gramberg in the institute of clinical and molecular virology in Erlangen (Germany) during which she studied the effect of TRIM5α polymorphism on LINE-1 retroelements sensing and restriction. She graduated in July 2023 and obtained a 1-year post-doctoral grant to study TRIM5α viral restriction mechanism in the same lab. Since June 2024, she joined Caroline’s team to work on the ERC-funded InVIRium project.
- Lenka Kocianova – Post-Doc

Lenka first joined the team for two short internships during her Bachelor’s degree in 2022 and 2023. Later, after completing two internships (one at the Institut Cochin in Paris and another at the Institute of Virology at the University of Marburg in Germany) during her Master’s in Infectiology at the University of Montpellier, she decided to rejoin us for her Master’s 2 internship. She is currently working with Caroline, Mary, Jimmy, and Olivier on a project exploring the interface between cellular signaling and the MX1 GTPase during influenza virus infection, aiming to contribute to a better understanding of the general antiviral role of this restriction factor.
The Interferon and Antiviral Restriction Lab, march 2025

The Interferon and Antiviral Restriction Lab, march 2024

From left to right : Issy Fernanda Hincapie Mena, Alice Trausch, Jimmy Cadènes, Elodie Bishop, Inken Junod, Ana-Luiza Chaves Valadao (ex IR), Marine Tauziet (ex IE), Olivier Moncorgé, Caroline Goujon, Mary Arnould, Fransisco Garcia de Gracia (ex post doc), Antoine Rebendenne.
The Interferon and Antiviral Restriction Lab, september 2022

From left to right: Francisco Garcia de Gracia, Caroline Goujon, Elodie Bishop (bottom row), Marine Tauziet (Top row), Joe McKeller, Olivier Moncorgé, Ana Luiza Chaves Valadão, Mary Arnould.
The Interferon and Antiviral Restriction Lab, Summer 2020.

From left to right: Francisco Garcia de Gracia, Olivier Moncorgé, Marine Tauziet, Joe McKellar, Mary Arnould, Ana Luise Chaves Valadão, Antoine Rebendenne, Boris Bonaventure.
The Interferon and Antiviral Restriction Lab in December 2019.

From left to right: A. Rebendenne, M. Arnould, F. Garcia de Gracia, M. Tauziet, O. Moncorgé, B. Bonaventure, J. McKellar, and C. Goujon.
The interferon and antiviral restriction lab in July 2019

From left to right: M. Arnould, O. Moncorgé, J. McKellar, M. Tauziet, F. Garcia de Gracia, B. Bonaventure, C. Goujon, S. Allahverdiyeva.
The interferon and antiviral restriction lab in March 2019

From left to right: B. Bonaventure, J. McKellar, C. Goujon, C. André, S. Allahverdiyeva, O. Moncorgé, M. Tauziet, M. Arnould
The interferon and antiviral restriction lab in March 2018

Spring 2018. From left to right: O. Moncorgé, W. Djilli, M. Tauziet, B. Bonaventure, C. Goujon, C. André, J. McKellar
The interferon and antiviral restriction lab in October 2017

The team at the IRIM Retreat 2017. From left to right: O. Moncorgé, B. Bonaventure, C. Goujon, M. Tauziet.
The interferon and antiviral restriction lab in July 2017

From left to right : R. Planès, C. Goujon, M. Tauziet, B. Bonaventure, O. Moncorgé, E. Partiot.
The interferon and antiviral restriction lab in September 2016

The team at the IRIM Retreat 2016. From left to right : B. Bonaventure, C. Goujon, O. Moncorgé, R. Planès.
Past team members/students
- Inken Junod – M2 student
 Inken is an M2 student from the Molecular life sciences Master at the University of Hamburg, Germany. She works with Olivier Moncorgé and Alice Trausch on the optimization of plasmid-based influenza virus rescue.
Inken is an M2 student from the Molecular life sciences Master at the University of Hamburg, Germany. She works with Olivier Moncorgé and Alice Trausch on the optimization of plasmid-based influenza virus rescue.
- Ana Luiza Chaves Valadao – Research Engineer, UM
 Ana studied Biological Sciences at State University of Rio de Janeiro. She has a master degree in Parasitology from Oswaldo Cruz Foundation where she worked in the development of a bivalent vaccine against yellow fever and dengue viruses. During her PhD, she focused her research in the role of Processing Bodies and Stress Granules during HIV replicative cycle. She did an intership, still during her PhD, at Institute of Human Genetics, in Montpellier, under the supervision of Monsef Benkirane, when they were interested in unveilling the activation pathway of HIV restriction factor SAMHD1. She started a post-doc in Brazil, working with HIV-1 latency and antiviral compounds against Zika virus. During this period, she was also teaching Genetics and Evolution for undergratuating students at Federal University of Rio de Janeiro. In 2016, she joined again the Institute of Human Genetics, now as a post-doc in Nadine Laguette team, working with Cancer Related Inflammation (MSDAvenir fellowship). After two years of contract, she became the Research Engineer of Laguette’s group. In April 2020, Ana has joined the Interferon and antiviral restriction lab to work on new antivirals against Flu (ERC PoC FluAttack Project), in collaboration with Olivier Moncorgé, and has also been involved in SARS-CoV-2 projects (ERC StG ANTIViR). Since 2023, she is a University of Montpellier Engineer and works on the InVIRium project.
Ana studied Biological Sciences at State University of Rio de Janeiro. She has a master degree in Parasitology from Oswaldo Cruz Foundation where she worked in the development of a bivalent vaccine against yellow fever and dengue viruses. During her PhD, she focused her research in the role of Processing Bodies and Stress Granules during HIV replicative cycle. She did an intership, still during her PhD, at Institute of Human Genetics, in Montpellier, under the supervision of Monsef Benkirane, when they were interested in unveilling the activation pathway of HIV restriction factor SAMHD1. She started a post-doc in Brazil, working with HIV-1 latency and antiviral compounds against Zika virus. During this period, she was also teaching Genetics and Evolution for undergratuating students at Federal University of Rio de Janeiro. In 2016, she joined again the Institute of Human Genetics, now as a post-doc in Nadine Laguette team, working with Cancer Related Inflammation (MSDAvenir fellowship). After two years of contract, she became the Research Engineer of Laguette’s group. In April 2020, Ana has joined the Interferon and antiviral restriction lab to work on new antivirals against Flu (ERC PoC FluAttack Project), in collaboration with Olivier Moncorgé, and has also been involved in SARS-CoV-2 projects (ERC StG ANTIViR). Since 2023, she is a University of Montpellier Engineer and works on the InVIRium project.
- Francisco Garcia de Gracia – Postdoc (ERC)
 Minibio: Francisco studied engineering in molecular biotechnology at the Universidad de Chile where his work focused on the characterization of the pre-integration complex of the murine leukemia virus in Prof. Monica Acevedo´s laboratory. He then did his PhD in microbiology at the Universidad de Chile-Universidad de Santiago and worked in Prof. Ricardo Soto-Rifo’s laboratory. His PhD thesis focused on the regulation of HIV-1 gene expression. Francisco has joined the lab in May 2019 and works on several aspects of the ERC ANTIViR project.
Minibio: Francisco studied engineering in molecular biotechnology at the Universidad de Chile where his work focused on the characterization of the pre-integration complex of the murine leukemia virus in Prof. Monica Acevedo´s laboratory. He then did his PhD in microbiology at the Universidad de Chile-Universidad de Santiago and worked in Prof. Ricardo Soto-Rifo’s laboratory. His PhD thesis focused on the regulation of HIV-1 gene expression. Francisco has joined the lab in May 2019 and works on several aspects of the ERC ANTIViR project.
- Joe McKellar – Postdoc (former PhD student, Montpellier university)
 After gaining a B.Sc. in Cellular Biology and Physiology from Limoges University, Joe did his Master at Montpellier University and a 6-month internship in the group. He then worked as a research assistant. He has obtained the French government MNERT scholarship to do his PhD in the lab and started in October 2018. Joe studies the mechanism of action of MX1 protein against influenza A virus under the supervision of Olivier and is specialized in cell bology and microscopy approaches. In 2021, Joe has obtained a FRM fellowship to do a 4th year. Joe has defended his PhD on the 17th of November 2022 and is spending a few more months in the lab as a postdoc.
After gaining a B.Sc. in Cellular Biology and Physiology from Limoges University, Joe did his Master at Montpellier University and a 6-month internship in the group. He then worked as a research assistant. He has obtained the French government MNERT scholarship to do his PhD in the lab and started in October 2018. Joe studies the mechanism of action of MX1 protein against influenza A virus under the supervision of Olivier and is specialized in cell bology and microscopy approaches. In 2021, Joe has obtained a FRM fellowship to do a 4th year. Joe has defended his PhD on the 17th of November 2022 and is spending a few more months in the lab as a postdoc.
- Boris Bonaventure – M2 student (2016), PhD student (Oct. 2016-Dec. 2020) and Postdoc (until Sept. 2021)
 Minibio: Boris studied at the ENS of Lyon. After internships at Oxford university and Harvard Medical School, Boris joined the lab for his third Master internship in Feb. 2016. He has obtained the French government MNERT scholarship to do his PhD in the lab and has started in October 2016. Boris is applying powerful, whole-genome CRISPR/Cas9 screens to the identification of new HIV-1 inhibitors. He has identified DDX42 as a new intrinsic inhibitor of HIV-1 and other viruses. He obtained a funding from the FRM for his 4th year of PhD and defended his thesis in Dec. 2020. Boris had then stayed for 9 months as a postdoc in the team and has now left to do a postdoc in the USA.
Minibio: Boris studied at the ENS of Lyon. After internships at Oxford university and Harvard Medical School, Boris joined the lab for his third Master internship in Feb. 2016. He has obtained the French government MNERT scholarship to do his PhD in the lab and has started in October 2016. Boris is applying powerful, whole-genome CRISPR/Cas9 screens to the identification of new HIV-1 inhibitors. He has identified DDX42 as a new intrinsic inhibitor of HIV-1 and other viruses. He obtained a funding from the FRM for his 4th year of PhD and defended his thesis in Dec. 2020. Boris had then stayed for 9 months as a postdoc in the team and has now left to do a postdoc in the USA.
- Marine Tauziet – Research assistant (ERC puis ANR)
 Minibio: Marine did a B.Eng. (DUT and Licence professionnelle, from La Rochelle and Montpellier university, respectively). She did a 4-months internship in the lab (Spring 2017) and was then recruited as a research assistant (AI) in July 2017. She has been recruited as an AI again in October 2017. After gaining a Master diploma, she has become an IE in the team. Marine works with Caroline on the characterization of a new antiviral interferon-stimulated gene. She will leave the team at the end of December 2022 and start a new position at IRCM.
Minibio: Marine did a B.Eng. (DUT and Licence professionnelle, from La Rochelle and Montpellier university, respectively). She did a 4-months internship in the lab (Spring 2017) and was then recruited as a research assistant (AI) in July 2017. She has been recruited as an AI again in October 2017. After gaining a Master diploma, she has become an IE in the team. Marine works with Caroline on the characterization of a new antiviral interferon-stimulated gene. She will leave the team at the end of December 2022 and start a new position at IRCM.
- Oriane Pourcelot. Spring 2021. M2 student, Montpellier University.
- Laura Navar. Spring 2020. L3 Pro student, IUT Montpellier.
- Sona Allahverdiyeva. Spring 2019. Master (M2) student, Montpellier university. Sona is doing a PhD in virology in Amsterdam.
- Charlotte Arpin-André, Research assistant (CNRS). Spring 2018- Spring 2019.
- Timothée Lalo-Deltour – BSc (L3) student, Montpellier university. Summer 2018 (7 weeks).
- Joe McKellar – Master (M2) student, Montpellier university (6 mois). Joe has done his PhD in the lab.
- Wassila Djilli – Master (M2) student, Strasbourg university. January-June 2018 (5 months).
 After a technical diploma followed by a B.Sc., Wassila was a Master student at Strasbourg University and did a 5-month internship in the group. Wassila worked with Boris on the identification and characterization of new interferon-induced HIV-1 inhibitors. Wassila is currently pursuing her studies to become a chemist/pharmacist.
After a technical diploma followed by a B.Sc., Wassila was a Master student at Strasbourg University and did a 5-month internship in the group. Wassila worked with Boris on the identification and characterization of new interferon-induced HIV-1 inhibitors. Wassila is currently pursuing her studies to become a chemist/pharmacist.
- Rémi Planès – Postdoc (Sidaction). Oct. 2015-Sept. 2017. Current email address: Remi.Planes(AT)ipbs.fr
Rémi did his PhD in Toulouse (INSERM U1043, CHU Purpan), under the supervision of Prof. E. Bahraoui. His thesis unravelled the role of HIV-1 Tat protein in TLR4 signalling. Rémi was then awarded a 1-year FRM fellowship to join Dr P. Guermonprez at the Centre for Molecular and Cellular Biology of Inflammation (CMCBI), King’s College London, where he studied the response of human dendritic cells to Plasmodium falciparum-infected red cells. Rémi was recently awarded a 2 year-fellowship by Sidaction to join the Interferon and antiviral restriction Lab and study the physiological role of MX2 and its potential involvement in innate immune signalling. Rémi worked in the lab from Oct. 2015 to Sept. 2017 and has now joined the lab of Etienne Meunier in Toulouse where he is studying the activation of the inflammasome by different pathogens.
- Emma Partiot, Master (M1) student. Paris Diderot University. June-July 2017 (2 months). Emma is doing her PhD in Raphaël Gaudin’s team.
- Leila Makrini, CNRS research assistant (AI). December-June 2017 (6 months).
- Marion Chateauraynaud, Master (M1) student. Montpellier university. Spring 2017 (2 months).
- Antoine Rebendenne, BSc (L3) student. ENS-Cachan & Paris Unversity. Summer 2016 (2,5 months) and Master (M2) student (8 months Dec. 2019-Juillet 2020), now a PhD student in the team.
- Océane Paris, Master (M1) student. Montpellier university. Spring 2016 (2 months).
- Boris Bonaventure, Master (M2) student. Ecole Normale Supérieure de Lyon. Spring 2020 (3 months). Boris has done his PhD in the team and a short postdoc.
Main publications and recent preprints (C. Goujon)
# corresponding authors
* contributed equally to the experimental work
(bold: lab members)
2023
- McKellar J., Arnaud-Arnould M., Chaloin L., Tauziet M., Arpin-André C., Pourcelot O., Blaise M., Moncorgé O.#, and Goujon C.# An evolutionarily conserved N-terminal leucine is essential for MX1 GTPase antiviral activity against different families of RNA viruses. Journal of Biological Chemistry, in press, 2023.
2022
- McKellar J., Arnaud-Arnould M., Chaloin L., Tauziet M., Arpin-André C., Pourcelot O., Blaise M., Moncorgé O.#, and Goujon C.# An evolutionarily conserved N-terminal leucine is essential for MX1 GTPase antiviral activity against different families of RNA viruses. Journal of Biological Chemistry, in press, 2022.
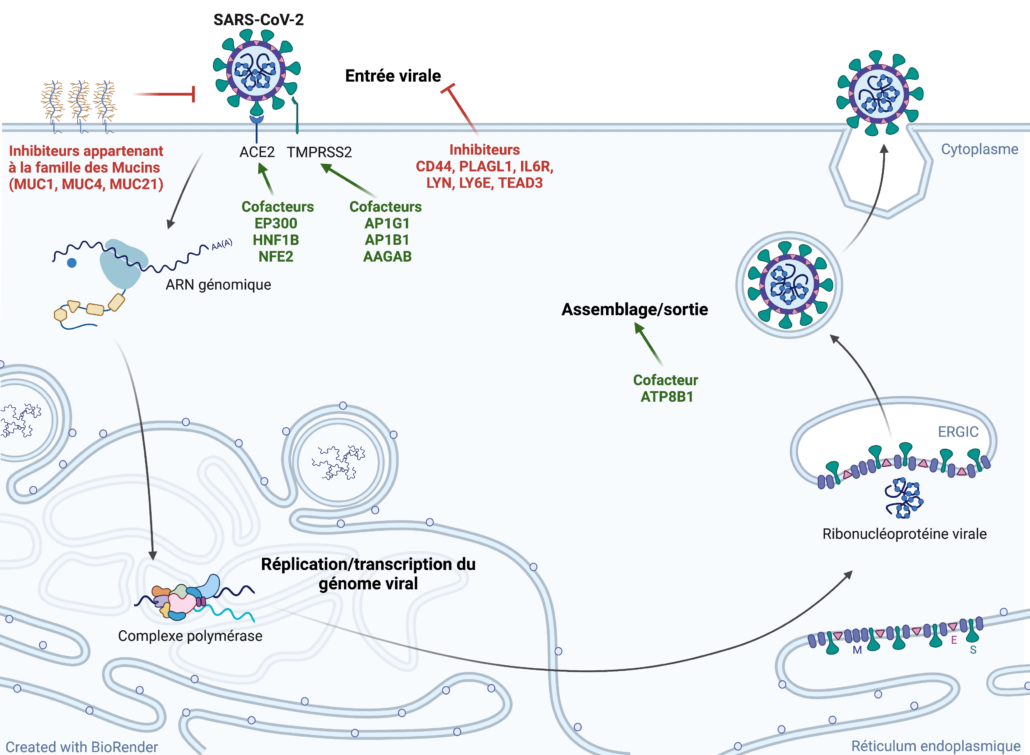
- Rebendenne A., Roy P., Bonaventure B.*, Chaves Valadão A.L.*, Desmarets L., Rouillé Y., Tauziet M., Arnaud-Arnould M., Giovannini D., Lee Y., DeWeirdt P., Hegde M., Garcia de Gracia F., McKellar J., Wencker M., Dubuisson J., Belouzard S., Moncorgé O., Doench J.G#, and Goujon C.# Bidirectional genome-wide CRISPR screens reveal host factors regulating SARS-CoV-2, MERS-CoV and seasonal HCoVs. Nature Genetics, 2022. 54(8):1090-1102
- Bonaventure B.#, and Goujon C.# DExH/D-box helicases at the frontline of intrinsic and innate immunity against viral infections. Journal of General Virology, 2022. 103(8). Review
2021
- Jouvenet N.#, Goujon C.#, Banerjee A.#. Clash of the titans: interferons and SARS-CoV-2. Trends in immunology, online Oct. 2021. doi: 10.1016/j.it.2021.10.009
- Arnaud-Arnould M., Tauziet M., Moncorgé O., Goujon C., Blaise M. Crystal structure of the TLDc domain of human NCOA7-AS. Acta Crystallographica Section F: Structural Biology Communications, 2021, 77(8).
- Bauby H., Ward C.C., Hugh-White R., Swanson C.M., Schulz R., Goujon C.# and Malim M.H.# HIV-1 Vpr Induces Widespread Transcriptomic Changes in CD4 + T Cells Early Postinfection. mBio, 2021 29;12(3):e0136921.
- Maarifi G., Lagisquet J., Hertel Q., Bonaventure B., Chamontin C., Fuchs K., Moncorgé O., Tauziet M., Mombled M., Papin L., Molès J.P. Bodet C., Lévèque N., Gross A., Arhel N., Nisole S., Van de Perre P., Goujon C., Blanchet F.P. Alarmin S100A9 restricts retroviral infection by limiting reverse transcription in human dendritic cells. EMBO Journal, 2021, 40(16):e106540.
- Rebendenne Antoine, Roy Priyanka, Bonaventure Boris*, Chaves Valadão Ana Luiza*, Desmarets Lowiese, Rouillé Yves, Tauziet Marine, Arnaud-Arnould Mary, Giovannini Donatella, Lee Yenarae, DeWeirdt Peter, Hegde Mudra, Garcia de Gracia Francisco, McKellar Joe, Wencker Mélanie, Dubuisson Jean, Belouzard Sandrine, Moncorgé Olivier, Doench John G.#, and Goujon Caroline#. Bidirectional genome-wide CRISPR screens reveal host factors regulating SARS-CoV-2, MERS-CoV and seasonal HCoVs. bioRxiv. https://www.biorxiv.org/content/10.1101/2021.05.19.444823v2
- Rebendenne A.*, Chaves Valadão A.L.*, Tauziet M.*, Maarifi G., Bonaventure B., Planès R., McKellar J., Nisole S., Arnaud-Arnould M., Moncorgé O.*#, and Goujon C.*# (2021). SARS-CoV-2 triggers an MDA-5 dependent interferon response which is unable to control replication in lung epithelial cells. Journal of Virology. https://jvi.asm.org/content/early/2021/01/28/JVI.02415-20
- Pila-Castellanos I., Molino D., McKellar J., Lines L., Da Graca J., Tauziet M., Chanteloup L., Mikaelian I., Meyniel-Schicklin L., Codogno P., Vonderscher J., Delevoye C., Moncorgé O., Meldrum E., Goujon C., Morel E., de Chassey B. Mitochondrial morphodynamics alteration induced by influenza virus infection as a new antiviral strategy. PloS Pathogens. 2021, DOI:10.1371/journal.ppat.1009340
2020
- Bonaventure B., Rebendenne A., Garcia de Gracia F. , Tauziet M., McKellar J., Chaves Valadão A.L., Courgnaud V., Bernard E., Briant L., Gros N., Djilli W., Arnaud-Arnould M., Parrinello H., Rialle S., Moncorgé O., and Goujon C.# (2020) A genome-wide CRISPR/Cas9 knock-out screen identifies the DEAD box RNA helicase DDX42 as a broad antiviral inhibitor. bioRxiv. doi: https://doi.org/10.1101/2020.10.28.359356
- Rebendenne A.*, Chaves Valadão A.L.*, Tauziet M.*, Maarifi G., Bonaventure B., Planès R., McKellar J., .Nisole S., Arnaud-Arnould M., Moncorgé O.*#, and Goujon C.*# (2020). SARS-CoV-2 replication triggers an MDA-5-dependent interferon production which is unable to efficiently control replication. bioRxiv. doi: https://doi.org/10.1101/2020.10.28.358945
2018
- Doyle T., Moncorgé O., Bonaventure B., Pollpeter D., Lussignol M., Tauziet M., Apolonia L., Catanese M.T., Goujon C.# and Malim M.H.# (2018). The interferon inducible isoform of NCOA7 inhibits endosome-mediated viral entry. Nature Microbiology, (12):1369-1376.
- Dicks MDJ, Betancor G, Jimenez-Guardeño JM, Pessel-Vivares L, Apolonia L, Goujon C, Malim MH. (2018) Multiple components of the nuclear pore complex interact with the amino-terminus of MX2 to facilitate HIV-1 restriction. PLoS Pathogens. 14(11):e1007408.
2016
- Dicks, M., Goujon, C., Pollpeter D., Betancor G., Apolonia L., Bergeron J.R., Malim M.H. (2016) Oligomerization requirements for MX2 mediated suppression of HIV-1 infection. Journal of Virology 90 (1), 22-32.
2015
- Doyle, T., Goujon, C., and Malim, M.H. (2015). HIV-1 and interferons: who’s interfering with whom? Nature Reviews Microbiology 13, 403-413.
- Goujon, C.#, Greenbury, R.A., Papaioannou, S., Doyle, T., and Malim, M.H.# (2015). A Triple-Arginine Motif in the Amino-Terminal Domain and Oligomerization Are Required for HIV-1 Inhibition by Human MX2. Journal of Virology 89, 4676-4680.
2014
- Goujon, C.#, Moncorge, O., Bauby, H., Doyle, T., Barclay, W.S., and Malim, M.H.# (2014). Transfer of the amino-terminal nuclear envelope targeting domain of human MX2 converts MX1 into an HIV-1 resistance factor. Journal of Virology 88, 9017-9026.
- Schaller, T., Bauby, H., Hué, S, Malim, MH, Goujon, C#. (2014) New insights into an X-traordinary viral protein. Frontiers in Microbiology. Apr 8;5:126.
2013
- Goujon, C., Moncorge, O., Bauby, H., Doyle, T., Ward, C.C., Schaller, T., Hue, S., Barclay, W.S., Schulz, R., and Malim, M.H. (2013). Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502, 559-562.
- Goujon, C.*, Schaller, T.*, Galao, R.P., Amie, S.M., Kim, B., Olivieri, K., Neil, S.J., and Malim, M.H. (2013). Evidence for IFNalpha-induced, SAMHD1-independent inhibitors of early HIV-1 infection. Retrovirology 10, 23
2012
- Schaller, T., Goujon, C., and Malim, M.H. (2012). AIDS/HIV. HIV interplay with SAMHD1. Science 335, 1313-1314.
Local
- L. Chaloin. 3D model of MX proteins. Inhibitors of SARS-CoV-2.
- M. Blaise. Structure-function analysis of NCOA7, DDX42 and MX proteins.
- L. Espert, IRIM, Montpellier. Autophagy, innate responses and viruses
- J.L. Battini, IRIM and V. Courgnaud, IGMM, Montpellier. Genetic screens in haploid cells.
- L. Briant, IRIM, Montpellier. Identification of anti-arboviruses ISGs. Inhibition of CHIKV by DDX42.
- D. Muriaux & C. Favard, IRIM, Montpellier. MX interactions with lipids.
- L. Picas, IRIM, Montpellier. MX interactions with lipids.
- A. Bourdin, CHU de Montpellier. Primary airway epithelia.
- J.M. Bellanger, CEFE. Antiviral activity of mushroom extracts.
- M. Moriel, CRBM. Inhibitors of SARS-CoV-2.
National
- Consortium ANRS MIE PEPR 3D-LUNGO
- M. Wencker, CIRI, Lyon. Potential modulation of immune responses by NCOA7 in mice.
- E. Ricci et P.E. Mangeot, CIRI, ENS Lyon. CRISPR/Cas9 genetic modification of primary cells using the Nanoblade techonology. Host responses to SARS-CoV-2.
- B. Reina San Martin, IGBMC, Strasbourg. Proteomics of HIV-1 reverse transcription complexes.
- E. Meunier and R. Planès, IPBS, Toulouse. Impact of NCOA7 on bacterial infections.
- C. Cougoule, IPBS, Toulouse. Organoid infection with SARS-CoV-2.
- S. Belouzard, Institut Pasteur Lille. Impact on MERS-CoV of human genes regulating SARS-CoV-2 and seasonal HCoVs.
International
- Consortium Horizon Europe APPEAL
- J. Doench, Broad Institute, USA. Genome-wide screens to identify SARS-CoV-2 regulators
- G. Kochs, Université de Freiburg, Allemagne. MX and innate immunity
- X. Saelens, Ghent university, Belgique. MX1 mode of action.
- M.H. Malim, King’s College London, Royaume-Uni. Early responses to HIV-1 infection.
- W.S. Barclay, Imperial College Lodon, Royaume-Uni. Study of influenza A virus.
- K. Peng, Research group of virus-host interactions, Wuhan Institute of Virology, Chinese Academy of Sciences. New dependency factors of HIV-1.
- J. Doench, Broad Institute, USA. CRISPR screens to identify SARS-CoV-2 regulators.
September 2024 : The team finally wins the Golden Pipet at the IRIM days !

April 2024 : Antoine Rebendenne receives the thesis award from the Société Française de Virologie at the JFV, in the « Emergent viruses» category.


December 2023 : Antoine Rebendenne awarded the Young Scientist Award from Pôle Biosanté of Montpellier University, in Infectious diseases and Immunity for his PhD work published in Nature Genetics. Bravo Antoine !
November 2023 : Antoine Rebendenne has brilliantly defended his PhD! Congrats, Antoine!!
November 2023 : Caroline Goujon is promoted DR2 Inserm.
July 2017: Caroline Goujon has been awarded an ERC Consolidator Grant 2022 for the InVIRium project (Investigating Virus-Host Interplay in Human Primary Models of Genetically Modified Respiratory Epithelium)


April 2023 : Joe McKellar awarded a PhD prize by the French Society for Virology at the Annual meeting in Paris. Congrats Joe!!


November 2022 : Publication in Journal of Biological Chemistry of the first, first-author article of Joe McKellar. An evolutionarily conserved N-terminal leucine is essential for MX1 GTPase antiviral activity against different families of RNA viruses.
17th of November 2022 : Joe McKellar has brilliantly defended his PhD! Congrats, Joe !!



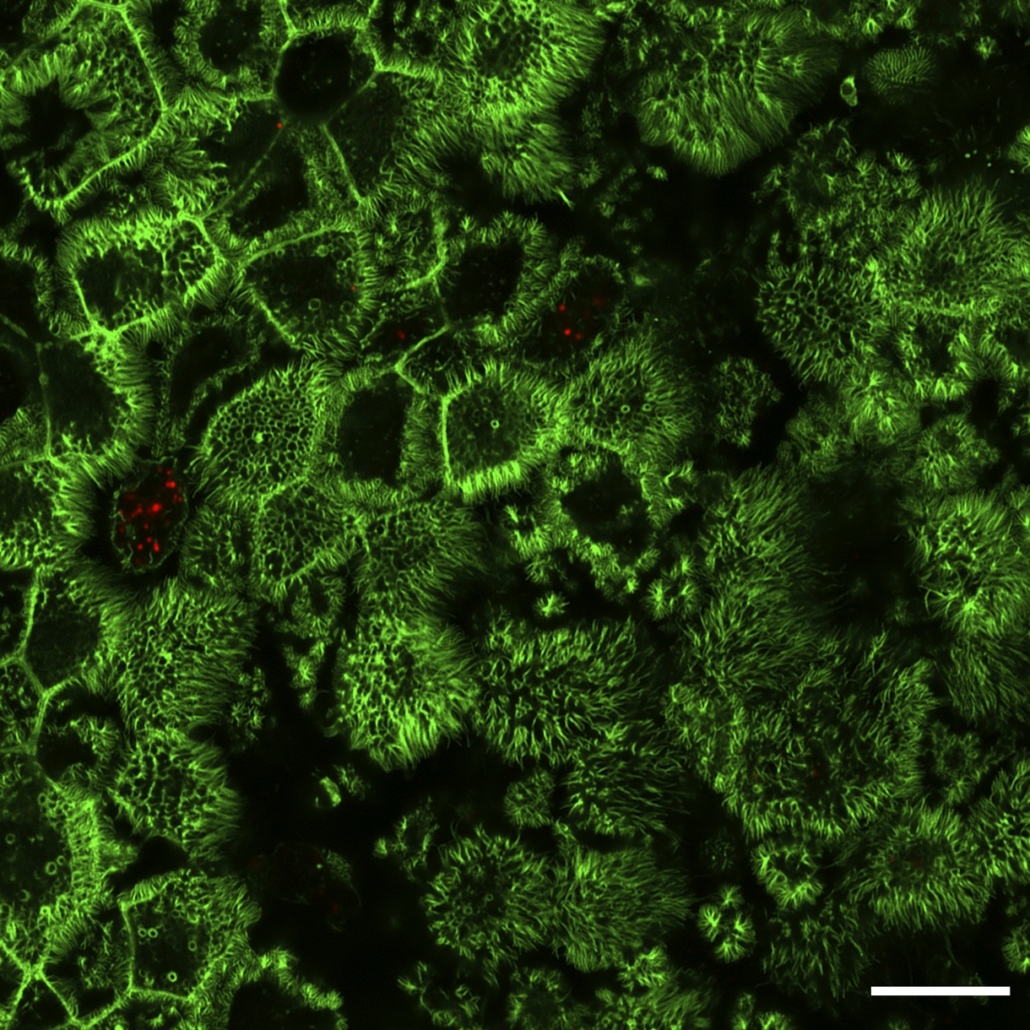
One of Joe McKellar’s microscopy image selected as an Image of dictinction at Nikon Small World 2022 Photomicrography competition
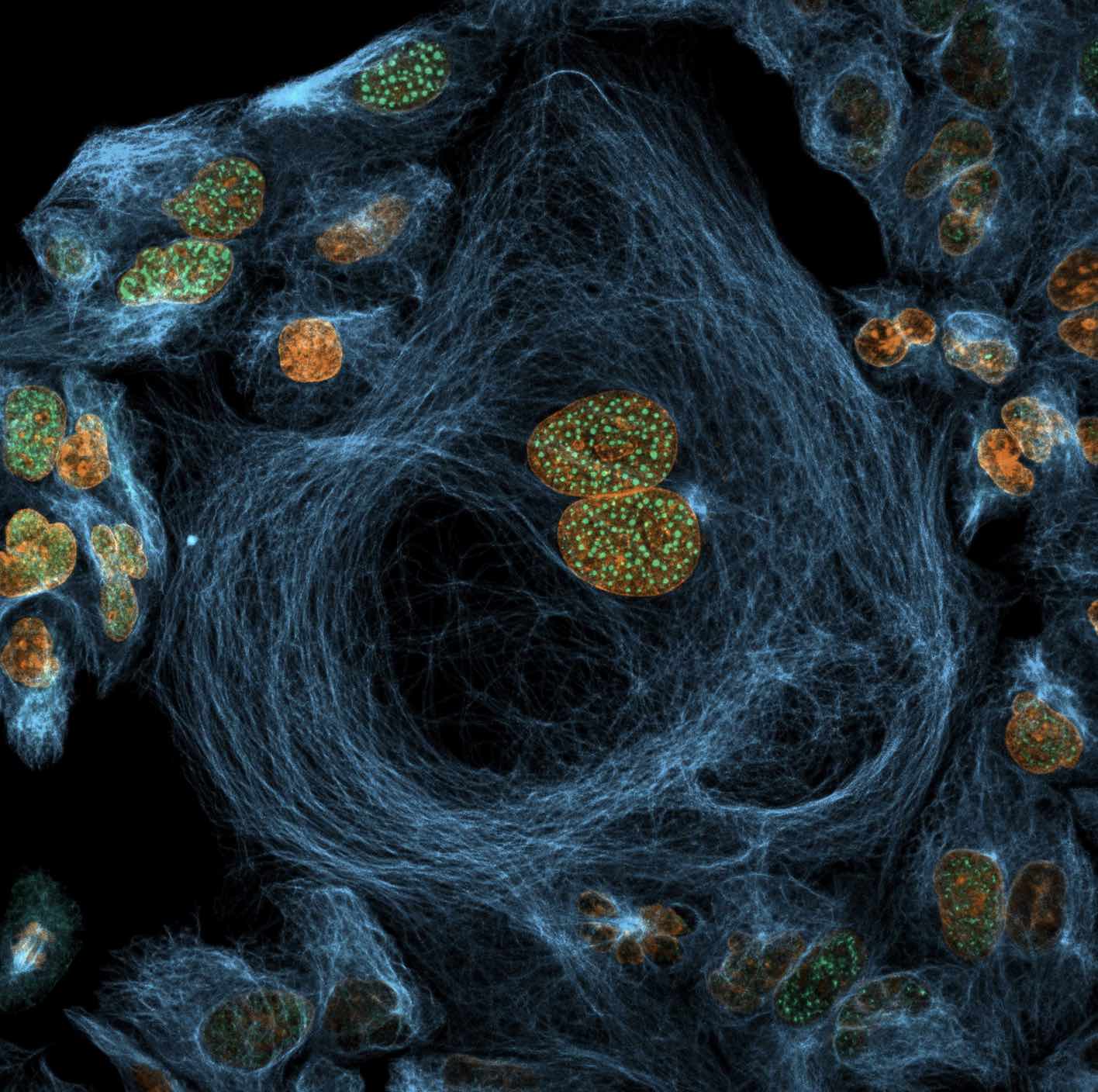
Nikon Small world 2022 Photomicrography Competition
August 2022 : The review from former PhD student, Boris Bonaventure, is out! DExH/D-box helicases at the frontline of intrinsic and innate immunity against viral infections
July 2022 : Our collaborative study on the identification of genes regulating SARS-CoV-2 replication in different model cell lines has been published in Nature Genetics : Bidirectional genome-wide CRISPR screens reveal host factors regulating SARS-CoV-2, MERS-CoV and seasonal HCoVs
Avril 2022 : Nouveau preprint! The N-terminal domain of MX1 proteins is essential for their antiviral activity against different families of RNA viruses
October 2021 : New forum par C. Goujon published in Trends in Immunology with N. Jouvenet and A. Banejee Clash of the titans: interferons and SASR-CoV-2
August 2021 : Our study revealing the tridimensional structure of NCOA7-AS TLDc domain, led by our collaborator Mickaël Blaise, is out! Crystal structure of the TLDc domain of human NCOA7-AS
May 2021 : Second preprint from the team on SARS-CoV-2 ! In collaboration with John Doench (Broad Institute, USA), we have performed bidirectional genome-wide CRISPR screens in cells that are physiologically relevant and naturally express ACE2 and TMPRSS2. We have identified both new dependency factors and new inhibitors of SARS-CoV-2! Some of these genes do also regulate MERS-CoV (data obtained by Jean Dubuisson’s team, Institut Pasteur Lille), and seasonal human coronaviruses HCoV-NL63 and HCoV-229E. Link towards tghe preprint here:
https://www.biorxiv.org/content/10.1101/2021.05.19.444823v2
January 2021 : the first publication from the team is out in Journal of Virology!
https://jvi.asm.org/content/early/2021/01/28/JVI.02415-20
In this study, we show a MDA-5 dependent interferon response in primary airway epithelia and model cell lines upon SARS-CoV-2 replication. However this interferon response, which arrives late, fails to control replication.
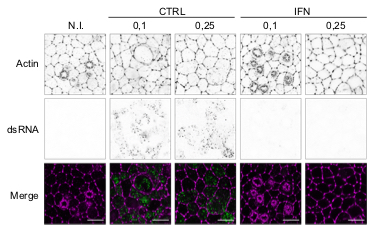
December 2020: The team was highlighted in the ‘Inserm magazine’ and on the inserm wbesite:
https://www.inserm.fr/en/research-inserm/live-from-our-labs/laboratory-in-motion/sars-cov-2-under-microscopes

October 2020: the first two preprints from the team are online!
The first preprint describes the identification of DDX42 as a broad antiviral inhibitor. Boris Bonaventure, in the team, performed whole-genome CRISPR screens which led to the identification of DDX42 as an intrinsic inhibitor of HIV-1, in various cell lines and primary cells. DDX42 inhibits viral DNA accumulation and is in close proximity with Capsid in infected cells. DDX42 is also capable of inhibiting retrotranspoition of Line-1 and infection with different retroviruses. Finally, we reveal that DDX42 is also a potent inhibitor of other pathogenic viruses, including SARS-CoV-2!
https://www.biorxiv.org/content/10.1101/2020.10.28.359356v1
All the team members participated in this work as well as Laurence Briant and Eric Bernard from IRIM (work on CHIKV) as well as Nathalie Gros from CEMIPAI (work on ZIKV). The library preparation and deep sequencing analysis was done by Hugues Parrinello and Stéphanie Rialle from Montpellier GenomiX.
In the second preprint, we have explored the host cell responses to SARS-CoV-2 both in primary human airway epithelia and in model cell lines. We observed a potent interferon induction, mainly due to sensing by MDA-5, but unable to control replication.
https://www.biorxiv.org/content/10.1101/2020.10.28.358945v1
This work was done in collaboration with cytokine specialists, Seb Nisole and Ghizlane Maarifi at the IRIM.
Below an image example acquired with a LSM880 microscope (Airyscan mode) of human airway epithelial cells (in green, Actin) infected by SARS-CoV-2 (red). Do admire the beauty of the ciliated cells seen from above for most of them ! Scale bar: 10 um, IF and image by Joe McKellar, infection by Olivier Moncorgé.
September 2020: First two ANR grants awarded to C. Goujon!
ANR RA-COVID for the CRISPR-TARGET-CoV project, aiming to identify new therapeutic targets against SARS-CoV-2, using whole-genome CRISPR screens, in collaboration with John Doench (Broad Institute, USA).
ANR PRC for the CAIPIRINAS (Control of type A Influenza virus Propagation and Immune Responses: Investigating the role of NCOA7-Alternative Start) project, in collaboration with M. Blaise and M. Wencker. This project aims to study NCOA4s mode of action in vitro, in cellulo and its role in vivo.

June 2020 : Antoine Rebendenne, ‘normalien agrégé’ from ENS-Paris Saclay awarded a PhD scholarship to do his PhD in the team.
The inserm presents the team’s projects on SARS-CoV-2 :
https://www.inserm.fr/actualites-et-evenements/actualites/quand-covid-19-reoriente-travaux-equipe-virologues
(in French)
Spring 2020 : Fundings obatined from MUSE, INSB and the Fondation CNRS for the team work on SARS-CoV-2.



March 2020 : While France is under lockdown, IRIM members, including our team work on SARS-CoV-2 research. In our team, C. Goujon, O. Moncorgé and M. Tauziet are in the lab everyday and perform experiments to better understand this new virus and potentially identify antiviral molecules. A. Chaves Valadao joins the COVID-19 team in April 2020, and A. Rebendenne at the end of the first lockdown.

March-April 2020: Team members working on covid19. From left to right: Marine Tauziet in our moleculat biology lab, Olivier Moncorgé in our Cat2 and Cat3 labs (CEMIPAI), Caroline Goujon in the cat3.
January 2020 : an ERC Proof of Concept grant (from the 3rd call 2019) awarded to C. Goujon for the FluAttack project led by O. Moncorgé (link: ERC website)


August 2019. Two innovation grants obtained from ‘la région Occitanie‘ : for the Antigrip Anticip project from the team and the Fongal Kombat Microbes project, led by Jean-Michel Bellanger (CEFE) in collaboration with Matteo Bonazzi’s team and our team.


September 2019: Celebratory drinks for the Region grants.
June 2019. Boris Bonaventure awarded a 4th year for his PhD by La Fondation pour la Recherche Médicale (FRM).

March 2019. Six lab members at the ‘Journées Francophones de Virologie’, ENS-Lyon and many other IRIM members! And second best poster prize for Boris Bonaventure: congrats, Boris!


November 2018. Publication in Nature Microbiology of our collaborative article with M. Malim’s team (King’s College London) describing a new antiviral ISG:
Doyle T., Moncorgé O., Bonaventure B., Pollpeter D., Lussignol M., Tauziet M., Apolonia L., Catanese M.T., Goujon C.# and Malim M.H.#. The interferon inducible isoform of NCOA7 inhibits endosome-mediated viral entry. Nature Microbiology, (12):1369-1376.
(# corresponding authors)
Link towards the ‘Behind the paper’ post from C. Goujon : Entry forbidden, a new antiviral ISG identified
June 2018. Joe McKellar, a Master student in the team, ranked 1st at the competitive exam from CBS2 to obtain the PhD scholarship from the French government (MRT).
Mars 2018 : Article featuring Caroline Goujon on the INSERM website.
September 2017 : After 2 years with us, Rémi Planès leaves the lab and moves back to Toulouse to start a new postdoc on the inflammasome. Best of luck for your future Rémi !


29th of September 2017: Farewell drinks and lunch at the IRIM.
July 2017: Caroline Goujon has been awarded an ERC Starting Grant for the ANTIViR project (Mechanisms of interferon-induced antiviral restriction and signalling)




July 2017: Celebration drinks at the institute for the accepted grants.
January 2017: Olivier Moncorgé has been awarded a 1-year renewal of his ANRS postdoctoral fellowship, to characterize new anti-HIV-1 inhibitors identified in whole-genome scale CRISPR/Cas9 genetic screens.

July 2016 : Caroline Goujon has obtained grants from Sidaction and the ANRS (for 2 and 3 years, respectively), for a collaborative project with Jean-Luc Battini (IRIM, ex-IGMM) and Valérie Courgnaud (IGMM) to identify MX2 cellular cofactors and other interferon-induced HIV-1 inhibitors using powerful genetic screens.


June 2016 : Boris Bonaventure, a Master student in the team, ranked 2nd at the competitive exam from CBS2 to obtain the PhD scholarship from the French government (MRT).
July 2015 : Rémi Planès has obtained a 2-year postdoctoral fellowship from Sidaction to join the team and study the potential role of human MX2 in innate immune signalling.


Opened: 2 Postdoctoral positions on innate immunity and respiratory viruses from 2024
We are recruiting 2 postdoctoral fellows on innate immunity and respiratory viruses to work on the ERC-funded InVIRium project, for up to 4 and 5 years, respectively:
– 1 postdoctoral fellow with a strong expertise in the fields of innate immunity and virology (experience in BSL-3 lab, and with respiratory viruses required). Experience in 3D-cultures of primary airway epithelia would be a massive plus.
– 1 postdoctoral fellow with skills in bioinformatics (scRNA-seq and NGS analyses) and in wet lab experiments, with expertise in molecular virology.
The InVIRium project aims at understanding the relationships between respiratory viruses and their host cells, using highly pertinent models of airway epithelia cultured at the air-liquid interface, with a major focus on respiratory viruses (coronaviruses, influenza viruses and respiratory syncytial virus) and on interferon-induced antiviral restriction.
The IRIM offers access to all the facilities required for cutting-edge research, including BSL2 and BSL3 labs, state-of-the-art cell imaging and microscopy facilities, flow cytometry and cell sorting, genomics, and proteomics platforms. The IRIM constitutes a friendly and international working environment, with staff and students coming from around 25 different countries. Montpellier is a lovely city in the South of France, 15 km away from the Mediterranean sea and offering an amazing quality of life. The 2 positions will be available from the beginning of 2024.
Candidates should send their application (CV and motivation, plus contact for 2-3 referees) by email to Caroline Goujon
At a glance
The interferon-induced antiviral state strongly inhibits viral replication in human cells, which means that our cells are capable of expressing powerful, natural antiviral inhibitors. Our team aims at understanding the mechanisms of the interferon-induced antiviral defences. Using powerful approaches (such as whole-genome CRISPR/Cas9 knock-out functional genetic screens), we aim at identifying new interferon-induced effectors, and then at characterizing their antiviral activity.
Funding

Past funding:




